Surgical site infections are among the most common healthcare-associated infections, accounting for up to 21.8 percent of HAIs, according to a study by Magill et al.1 SSIs not only cause increased morbidity and mortality for patients but also increase the cost of delivery of care due to hospital readmissions, increased length of stay,2, 3 and operative revisions, such as removal of hardware in orthopedic surgery infections.4 Up to 60 percent of SSIs are preventable and are penalized in the Value-Based Purchasing and Hospital Acquired Condition Reduction Programs.5 These conditions are not reimbursed by Medicare and include SSI following certain orthopedic procedures as well as other surgical procedures.6
This content is sponsored by Eloquest Healthcare.
Although the Institute for Healthcare Improvement, The Joint Commission and others have made recommendations leading to successful interventions to improve patient outcomes and reduce infection risk,7, 8 the prevalence and high cost of infectious complications warrant continuing effort to prevent hospital-acquired wound infections in surgical wounds and wounds caused by device insertion.
One area of active research is antimicrobial dressings with the purpose of protecting the wound, promoting an optimal healing environment, and reducing the risk for potential microbial growth under the dressing.
Preoperative skin antisepsis is recommended to reduce the risk of infection, but the skin's endogenous flora — the primary pathogen source for SSI — quickly regenerate.9 The use of antiseptic dressings can help reduce regrowth of bacteria on the skin.10 Postoperative dressings ideally help protect the wound against infection,11 and newer types of dressings have incorporated antimicrobial agents in pursuit of this goal. Although Staphylococcus is thought to be the major pathogen in SSIs, other pathogens are also frequently found in wounds, suggesting that an antimicrobial choice should include coverage for both gram-positive and gram-negative bacteria.12 Two commonly used antimicrobial agents used in antiseptic dressings are silver-based products and CHG.
CHG has been shown in multiple studies to be an effective antiseptic. CHG molecules have a positive charge, binding strongly with negative charges in the cell walls, causing cell death. The broad-spectrum activity of CHG includes an antiseptic effect against bacteria and yeast.13 CHG has also been found to be effective as part of a prevention bundle. Schweizer et al14 found that the use of CHG bathing, intranasal mupirocin and antibiotic prophylaxis prior to surgery led to a decrease in complex S. aureus wound infections.
The effects of CHG on microbial growth under dressings has also been studied. In a healthy human study, Bashir and colleagues10 found that CHG was effective in suppressing bacterial growth under occlusive dressings.
The ReliaTect® Post-Op Dressing with CHG was designed to provide many of the components of the ideal postoperative dressing, as described by Dumville et al15 in a Cochrane Review. These attributes include the ability to absorb and contain exudate without leakage, impermeability to water and bacteria, suitability of use with different types of wound closures, avoidance of wound trauma during dressing changes, and lower frequency of required dressing changes.11,16,17 In addition to these dressing characteristics, the ReliaTect® PostOp Dressing with CHG prevents external contamination of the wound through the functions of two different layers: the outer film layer and the adhesive layer. The outer film layer serves as a barrier that is impermeable to external contaminants, including fluids (waterproof), bacteria, viruses and yeast.18 The inner adhesive layer contains CHG.
In vitro tests have demonstrated that ReliaTect® Post-Op Dressing with CHG inhibits microbial colonization within the dressing.18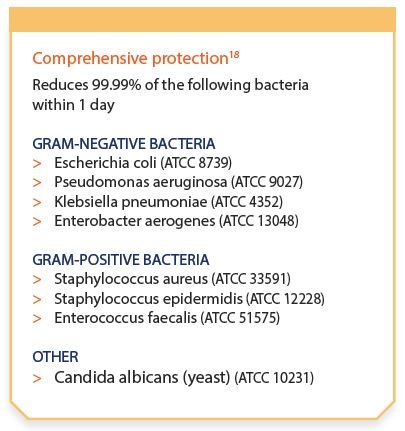
This antimicrobial effect lasts throughout the seven-day recommended wear time and provides a minimum of four-log reduction against a wide variety of clinically relevant microorganisms for up to seven days. The antimicrobial action is also rapid; ReliaTect® Post-Op Dressing with CHG reduces 99.99 percent of clinically relevant bacteria within one day.18
In addition to its antimicrobial benefits, the acrylic adhesive is absorptive. The dressing can absorb light to moderate amount of blood, perspiration and exudates, but is not designed for large amounts of fluid absorption.
ReliaTect® Post-Op Dressing with CHG is transparent, providing visualization of the surgical wound site for inspection and assessment; this feature may reduce the need for dressing changes and associated costs. Transparency helps facilitate daily, direct observation of the surgical site, which is the "gold standard" for SSI detection.19 The dressing is breathable, allowing for oxygen and moisture vapor exchange, yet is impermeable to external contaminants. The vapor transmission rate is greater than the absorption rate, and this dynamic moisture management can help the dressing to remain securely adhered in the presence of exudate and other fluids.18
ReliaTect® Post-Op Dressing with CHG has a wear time of up to seven days, minimizing the need for dressing changes.18 It is noncytotoxic, non-irritating to the skin, and the material is flexible, providing skin-friendly contact that conforms to bodily contours.
ReliaTect® Post-Op Dressing with CHG is offered in two sizes to accommodate different surgical wound sizes: 8 cm x 15 cm (3.2 in x 5.9 in) and 10 cm x 25 cm (3.9 in x 9.8 in).
References
1. Magill, S. S., Edwards, J. R., Bamberg, W., et al. (2014) Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med, 370(13), 1198-1208. doi:10.1056/NEJMoa1306801.
2. Kirkland, K. B., Briggs, J. P., Trivette, S. L., Wilkinson, W. E., & Sexton, D. J. (1999). The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol, 20(11), 725-730. doi:10.1086/501572
3. Digiovine, B., Chenoweth, C., Watts, C., & Higgins, M. (1999). The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med, 160(3), 976-981. doi:10.1164/ajrccm.160.3.9808145
4. Cai, J., Karam, J. A., Parvizi, J., Smith, E. B., & Sharkey, P. F. (2014). Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a casecontrol study. J Arthroplasty, 29(6), 1098-1100. doi:10.1016/j.arth.2013.11.012
5. Ban, K. A., Minei, J. P., Laronga, C., et al. (2017). American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg, 224(1), 59-74. doi:10.1016/j.jamcollsurg.2016.10.029
6. Centers for Medicare & Medicaid Services. (2015). Hospital-Acquired Conditions. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ HospitalAcqCond/Hospital-Acquired_Conditions.html
7. Office of Disease Prevention and Health Promotion. Health care quality and patient safety overview. Available at: https://health.gov/hcq/prevent-hai.asp
8. The Joint Commission. The Joint Commission's Implementation Guide forb NPSG.07.05.01 on Surgical Site Infections: The SSI Change Project. Available at: https://www.jointcommission.org/assets/1/18/Implementation_Guide_for_NPSG_SSI.pdf
9. Mangram, A. J., Horan, T. C., Pearson, M. L., Silver, L. C., & Jarvis, W. R. (1999).
Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease
Control and Prevention (CDC) Hospital Infection Control Practices Advisory
Committee. Am J Infect Control, 27(2), 97-132.
10. Bashir, M. H., Olson, L. K., & Walters, S. A. (2012). Suppression of regrowth of normal skin flora under chlorhexidine gluconate dressings applied to chlorhexidine gluconate-prepped skin. Am J Infect Control, 40(4), 344-348. doi:10.1016/j.ajic.2011.03.030
11. National Collaborating Centre for Women's and Children's Health. (2008). Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. Available at: https://www.nice.org.uk/guidance/cg74
12. Abboud, E. C., Settle, J. C., Legare, T. B., Marcet, J. E., Barillo, D. J., & Sanchez, J. E. (2014). Silver-based dressings for the reduction of surgical site infection: review of current experience and recommendation for future studies. Burns, 40 Suppl 1, S30-S39. doi:10.1016/j.burns.2014.09.011
13. Edmiston CE Jr, Bruden B, Rucinski MC, Henen C, Graham MB, Lewis BL. Reducing the risk of surgical site infections: does chlorhexidine gluconate provide a risk reduction benefit? Am J Infect Control. 2013;41(5 Suppl):S49-55. doi:10.1016/j.ajic.2012.10.030. Review. PubMed PMID: 23622749.
14. Schweizer, M. L., Chiang, H. Y., Septimus, E., et al. (2015). Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA, 313(21), 2162-2171. doi:10.1001/jama.2015.5387
15. Dumville, J. C., Gray, T. A., Walter, C. J., et al. (2016). Dressings for the prevention of surgical site infection. Cochrane Database Syst Rev, 12, CD003091. doi:10.1002/14651858.CD003091.pub4.
16. British Medical Association and Royal Pharmaceutical Society of Great Britain. (2011). British National Formulary (BNF): Appendix 8: wound management products and elasticated garments. Available at: https://www.bnf.org/products/bnf-online/
17. Goldman, M.P., Fronek, A. (1992). Consensus Paper on Venous Leg Ulcer. The Journal of Dermatologic Surgery and Oncology, 18(7), 592-602. doi:10.1111/j.1524-4725.1992.tb03513.x
18. Eloquest Healthcare. (2016). ReliaTect® Post-Op Dressing with CHG Instructions for Use. Data on file.
19. Anderson, D. J., Podgorny, K., Berrios-Torres, S. I., et al. (2014). Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol, 35(6), 605-627. doi: 10.1086/676022
MTR-MKT-000345-A
More articles on infection control:
Dayton VA officials admit to not disclosing patient's MRSA infection
40% of new parents show depressive symptoms at newborns' discharge
4 thoughts on using hand hygiene protocols to reduce the threat of sepsis
