Infection with the pathogen Clostridium difficile is a significant source of healthcare-associated infections and estimated to account for 12 percent of all HAIs in the U.S.1 C. diff infection is the leading cause of gastroenteritis-associated death and its incidence appears to be on the rise.2 Statistics from 2007 reveal that 14,000 deaths were attributed to CDI while similar data from 2011 estimate 29,000 deaths were CDI-mediated.2
This content is sponsored by Cepheid
Rapid identification of C. diff is an integral part of the battle against CDI. The development of polymerase chain reaction or nucleic acid amplification testing of stool specimens for C. diff has been a critical step forward in this effort to increase the rapidity, sensitivity and specificity of diagnosis. PCR testing has been shown to have a sensitivity ranging from 94 to 99 percent in the detection of toxigenic C. diff organism.3-5 Multiple studies demonstrate that the sensitivity of PCR testing for C. diff exceeds that of other testing modalities, including glutamate dehydrogenase, toxin enzyme immunoassay, and cell culture cytotoxin neutralization assay.3,6,7 As facilities transition to the more sensitive PCR testing, they may observe an increased incidence of C. diff, prompting some to wonder whether PCR might be too sensitive. This concern has raised three main questions: 1) Does PCR testing lead to over-diagnosis and over-treatment of C. diff?, 2) Does PCR lead to unnecessary isolation and contact precautions?, and 3) Does PCR testing lead to unnecessarily increased healthcare costs?
Over-diagnosis and Over-treatment of CDI
Most experts agree that the diagnosis of CDI should not be based on laboratory findings alone.3,7 A multifactorial approach that includes the patient’s clinical status (e.g., frequency of diarrhea), individual risk factors, imaging and laboratory findings independent of C. diff testing should be used in diagnosing a patient with CDI. While a large study demonstrated that positive PCR results correlated well with true CDI 3, a positive PCR result in the absence of other supporting clinical or laboratory findings does not unilaterally indicate CDI. Avoiding over-diagnosis with PCR, and therefore over-treatment, can be accomplished when PCR results are interpreted in the larger clinical context and by limiting testing to those patients with liquid/unformed stools.
Reliable identification of toxigenic C. diff is, however, an important piece of the diagnostic puzzle. Higher sensitivity results in increased pathogen detection and fewer missed cases. A recent British study of over 1,000 stool samples from two acute care facilities found that GDH/EIA testing missed 16.2 percent of CDI cases.3 These undiagnosed cases pose a significant threat for C. diff transmission if not identified and handled with the appropriate contact precautions.
Expediting diagnosis can also mitigate transmission risk. On-demand PCR testing can provide results in less than two hours, reducing delays in treatment that might lead to pathogen transmission.9 Kundrapu, et al. showed that expediting testing reduced delays in treatment of CDI, potentially reducing transmission risk, particularly if those patients are not placed in isolation.9 One study looking at CDI rates noted a decrease from 52 percent of hospital-onset, healthcare facility-associated CDI rates to 16 percent in the six months after they implemented PCR testing instead of EIA.10
PCR Testing and Isolation
The practice of implementing transmission-based precautions for patients with known infectious agents has become a mainstay of modern healthcare delivery. The CDC has issued recommendations for isolating patients with a variety of different infectious agents, including active CDI, as well as those patients with a history of previous infection/colonization or CDI.11 One study from a hospital in California documented that over a one-year period, 18.1 percent of hospital days were isolation days and that CDI, along with methicillin-resistant Staphylococcus aureus infections, were responsible for 75.5 percent of all hospital isolation days in their facility.10
There are two sides to the issue of isolation. If contact precautions and isolation of patients with C. diff are not implemented in a timely manner, the risk of transmission is significantly increased. Conversely, unnecessary isolation should be avoided when possible. The costs of isolating patients including the costs of personal protective equipment, such as gowns and gloves and labor costs, are substantial. Expedited testing with modalities, such as PCR-based C. diff testing, can reduce delays in isolation of patients with true CDI and minimize unnecessary isolation in C. diff negative patients.9
The Economics of PCR Testing
The economic burden of CDI is staggering. A study published in 2015 estimated the costs related to CDI just in acute care facilities in the U.S. to be $4.8 billion.2 The ability to rapidly and reliably diagnose CDI with PCR has been linked with downstream cost savings.3 In their 2009-2010 study, Catanzaro and Cirone demonstrated a significant decrease in isolation days, tests ordered, and metronidazole treatment in patients screened for CDI with PCR as compared with EIA.10 Healthcare-associated CDI decreased from 4.4 per 10,000 patient days to 0.9 per 10,000 patient days when PCR testing was utilized.10 These reductions can all translate into financial savings.
Given that every missed CDI case may bring associated costs, sensitive tests can be useful in lowering cost burdens by reducing the frequency of missed cases. In cost-benefit analysis of different testing strategies for hospital-associated CDI, Schroeder, et al. found that on-demand PCR testing was the most cost-effective method (Figure 1).8 Although PCR-based tests may be more expensive than other methods, these costs are minimal relative to those resulting from increased transmission rates and extended stays associated with testing by other methods, such as stand-alone EIAs.6
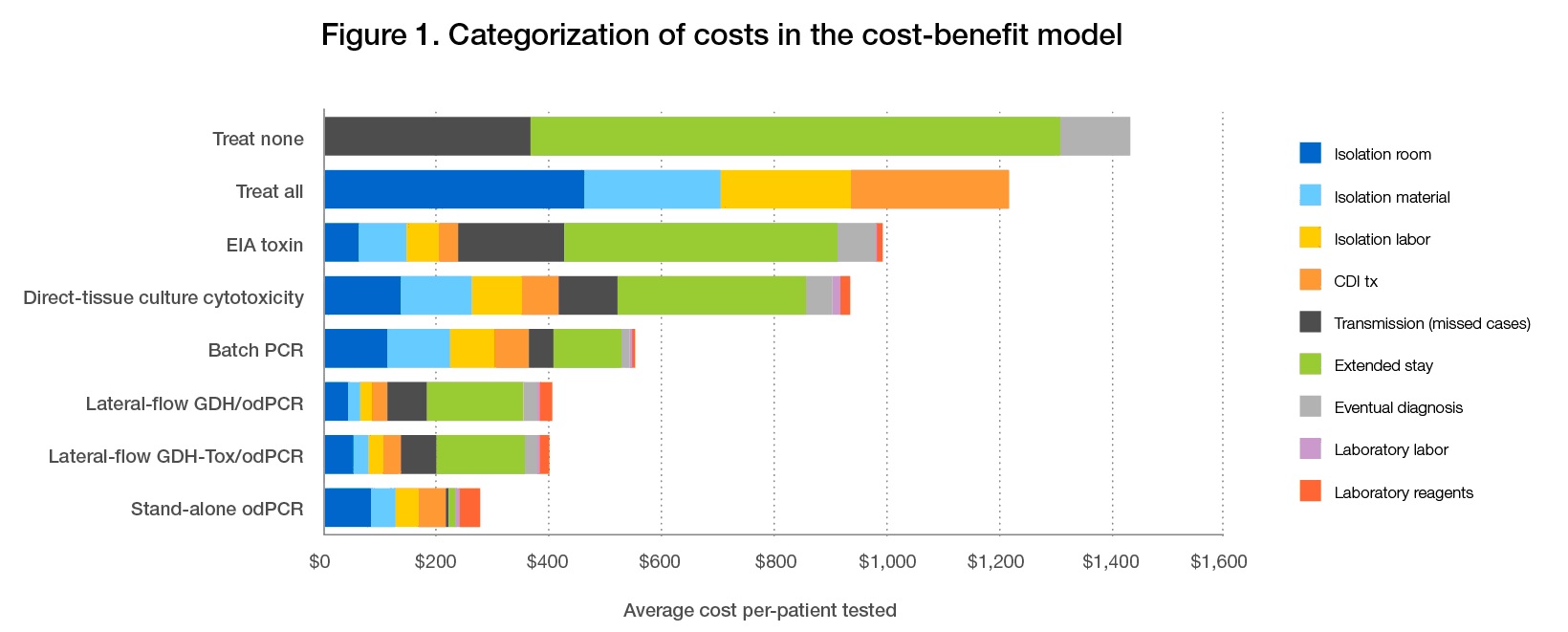
Figure 1. Despite a higher cost per test, when compared with other testing methodologies stand-alone PCR testing was associated with the lowest average cost per patient. EIA = enzyme immunoassay; PCR = polymerase chain reaction; GDH = glutamate dehydrogenase; OD = on demand. Reprinted with permission from the Journal Clinical Microbiology.8
There has been concern that increased C. diff rates potentially resulting from using sensitive PCR testing could lead to penalties imposed by CMS once CDI rates become part of the CMS value-based purchasing program in 2017. It is important to note, however, that the CDC performs risk-adjusted calculations to control for testing modality (EIA, PCR, etc.) and its inherent sensitivity and specificity before submitting rates to CMS.12 Facilities, therefore, will not incur penalties for using more sensitive testing modalities simply on the basis of their test of choice.
Looking Forward
In recent years, there has been an emergence of a new strain of C. diff, bearing one of several different names dependent on the testing method used to type it: PCR ribotype 027, North American pulsed-field gel electrophoresis type 1, or restriction endonuclease analysis type B1. A study published in July of 2015 demonstrated that infection with this strain independently predicted CDI disease severity and mortality.13 As the landscape of CDI evolves, it may become even more critical to employ a rapid and sensitive means of testing for C. diff like PCR.
References
1. Bamberg WM, et al. Multistate point prevalence survey of health care-associated infections. N Engl J Med. 2014;(370):1198-208.
2. Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;(372):825-34.
3. Berry N, et al. Real-time polymerase chain reaction correlates well with clinical diagnosis of Clostridium difficile infection. J Hosp Infect. 2014;(87):109-14.
4. Cumpio J, et al. Clostridium difficile testing in the clinical laboratory by use of multiple testing algorithms. J Clin Microbiol. 2010;(48):889-93.
5. Davis T, et al. Impact of strain type on detection of toxigenic Clostridium difficile-comparison of molecular diagnostic and enzyme immunoassay approaches. J Clin Microbiol. 2010;(48):3719-24.
6. Balada-llasat JM, et al. Detection of toxigenic Clostridium difficile: comparison of the cell culture neutralization, Xpert C. difficile, Xpert C. difficile/Epi, and Illumigene C. difficile assays. J Clin Microbiol 2012; 50:1331-1335.
7. Humphries RM, et al. Performance of Clostridium difficile toxin enzyme immunoassay and nucleic acid amplification tests stratified by patient disease severity. J Clin Microbiol 2013; 51(3):869-873.
8. Banaei N, et al. Economic evaluation of laboratory testing strategies for hospital-associated Clostridium difficile infection. J Clin Microbiol. 2014;(52):489-96.
9. Donskey CJ, et al. Easily modified factors contribute to delays in diagnosis of Clostridium difficile infection: a cohort study and intervention. J Clin Microbiol. 2013;51(7):2365-70.
10. Catanzaro M, et al. Real-time polymerase chain reaction testing for Clostridium difficile reduces isolation time and improves patient management in a small community hospital. Am J Infect Control. 2012;(40):663-666.
11. Chiarello L, et al. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control 2001;35(10)2:S65-S164.
12. Dudeck MA, et al. Risk adjustment for healthcare facility-onset C. difficile and MRSA bacteremia laboratory-identified event reporting in NHSN. 12 March 2013. Available from: http://www.cdc.gov/nhsn/pdfs/mrsa-cdi/Risk/Adjustment-MRSA-CDI.pdf. Accessed 29 October 2015.
13. Kiel MJ, et al. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clin Infect Dis 2015;61(2):233-241.
