Throughout the healthcare system – from pharmacy benefit managers and managed care organizations to government agency formularies and hospital and health networks – early adopters are embracing the methodology and technology to improve drug decision making and safety.
These forward thinkers are reexamining current standard practices regarding formulary choices and recognizing that a thorough analysis of the most current post-approval drug side effect data should be obligatory. Organizations can no longer overlook the reporting of adverse side effects when a medication is used by a broad range of real-world patients. Often, analysis is necessary to uncover the true depth of side effects and new drug risks that never arose during clinical trials. During the post-approval period, three-times more adverse drug reactions are identified than during clinical trials.
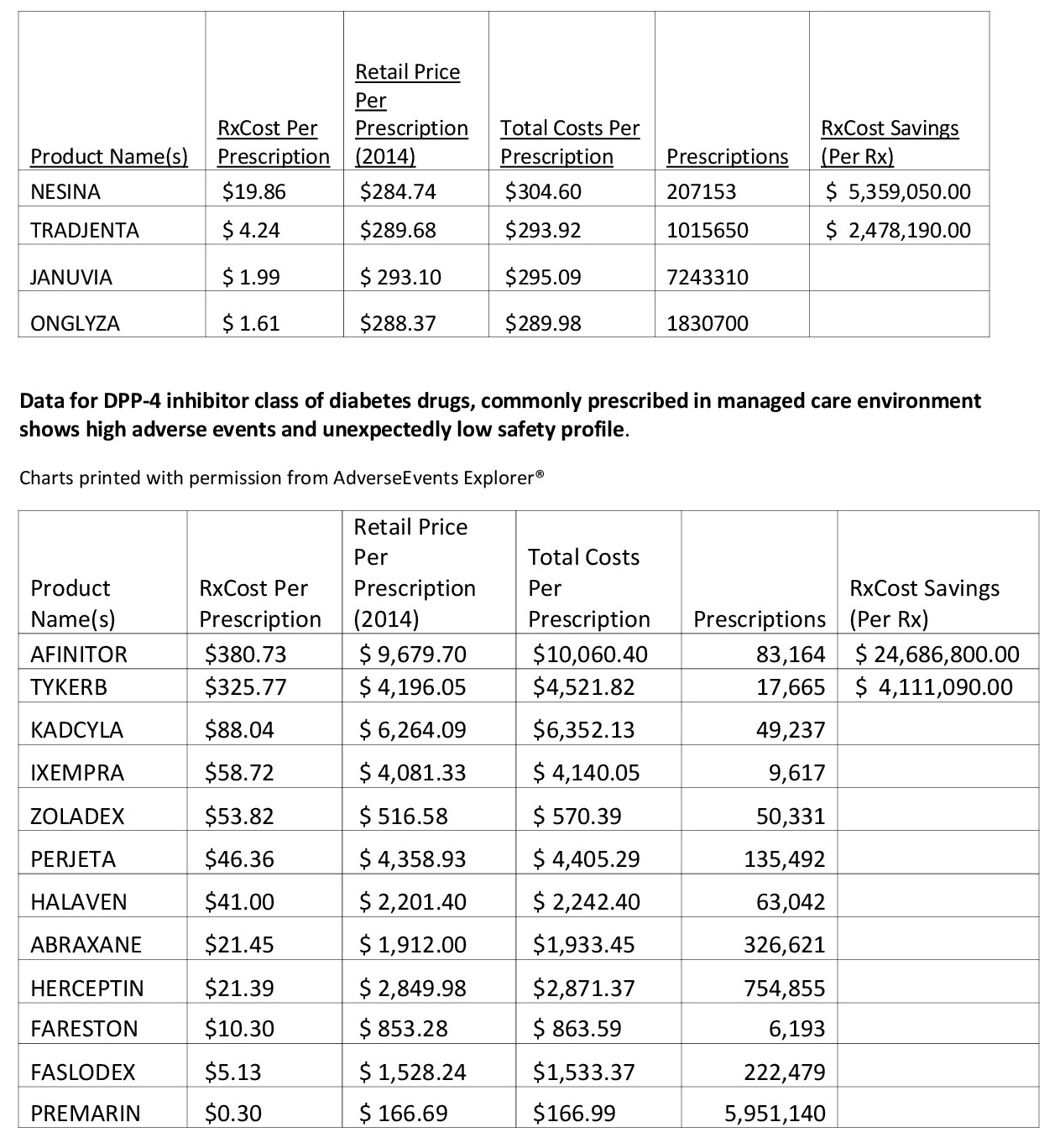
Healthcare providers and payers should strive to employ the most comprehensive information about a drug’s true cost and safety by assessing these real-world data and making decisions based the costs of actual adverse side effects of a drug (e.g., injury, hospital admission/readmission, disability and/or death). At the regional Texas healthcare group Memorial Hermann, this belief in adverse events analytics is strong and permeates the organization.
The reasons why this analysis of adverse side effect is essential are compelling:
– $4.7 billion in reported adverse event side effect costs in 2013
– Suspected $25 billion in true costs if unreported adverse drug events are considered
– 148,000 hospital admissions or readmissions as a result of adverse drug events
– Making more informed drug choices using comprehensive and actionable adverse drug events data leads to improved patient safety and outcomes and reduced costs.
Therefore, calculating a medication’s “downstream” costs once adverse events risks are considered can result in significant and necessary changes to a healthcare entity’s medication recommendations. For example, certain drugs that are frequently prescribed by healthcare professionals in hospitals or managed care organizations can cost the system much more than anticipated if the level or severity of adverse events are more than anticipated.
To address this issue, organizations must continue to advance and implement state-of-technology analytics methodology. Thereby, more health care professionals will be able to assess adverse drug event and healthcare economic data to help determine a drug’s total medical cost and the long-term economic impact of prescribing a particular drug. This task can be accomplished by the following steps:
– Choose or build a software system that includes data gathered from the FDA Adverse Events Reporting System (FAERS) and a process for refining this data, which is frequently in need of refinement. For example, a drug such as Ambien could be input many ways, with spelling errors, at different dosages and release formulations, but the analysis may be more helpful if this information was corrected and combined.
– Get the latest information about recent adverse events by soliciting this information from the FDA using the Freedom of Information Act. Typically, the FDA will receive data regarding adverse events, but there may be a three-to-nine month lag as to when it is input into FAERS. This gap provides yet another opportunity for severe adverse events to escalate in the public domain without acknowledgement about increased risks and costs to the system.
– Develop or utilize the analytics component of your system, whether it is customized to an organization or purchased from an independent company. These algorithms should produce actionable outputs that guide healthcare professionals and formularies in making better-informed decisions about drugs. Ideally, the system will:
– assess and compare medicines with a particular class, indication or mechanism of action and rate both the amount and severity of adverse reactions and their costs
– reveal the costs related to serious adverse events and outcomes per patient
– identify the specific adverse drug reactions that are driving increased costs
– compare cost per patient with the average cost of other drugs in an indication, class or mechanism of action
– show both on- and off-label costs
– Educate users in your organization about the system so that it is a living, breathing tool that is embraced and constantly improved upon.
Tomorrow’s world of healthcare will see clearly that the era of big data improving drug safety has arrived. Pioneers will demonstrate to others that changing inherent practices isn’t as daunting as it seems and open the door to a wealth of empowering information. Most importantly, patient lives will be saved by improved drug decision making.
The views, opinions and positions expressed within these guest posts are those of the author alone and do not represent those of Becker’s Hospital Review/Becker’s Healthcare. The accuracy, completeness and validity of any statements made within this article are not guaranteed. We accept no liability for any errors, omissions or representations. The copyright of this content belongs to the author and any liability with regards to infringement of intellectual property rights remains with them.
