Ultrasound probes can be contaminated after patient use
Ultrasound probes contact a variety of hazards during use, leading to microbial contamination even with the use of a sheath. Over 90% of transvaginal ultrasound probes can be contaminated with bacteria after use.4,5 Studies show that even after low-level disinfection with manual wipes and sprays, probes can be contaminated with viruses and bacteria, including pathogens that cause sexually transmitted infections like human papillomavirus and Chlamydia trachomatis.4,6,7 A study of ultrasound probes in emergency and ICU settings found that almost 50% were contaminated with pathogens, including Staphylococcus and Enterococcus bacteria.8
A landmark epidemiological study by the health authority in Scotland reported an increased risk of infection in the 30 days following an endocavitary ultrasound scan, where low-level disinfection was the practiced standard of care.9 The study followed almost 1 million patient journeys retrospectively through linked national patient datasets between 2010 and 2016. The Scottish government has mandated high level disinfection (HLD) for semi-critical probes including endocavitary probes since 2016.10
Standard precautions dictate that every patient must be assumed to be infectious to help break the chain of infection transmission. In medical device disinfection, this means applying the Spaulding classification to the probe before use on a patient (Figure 1).11 Spaulding forms the basis of National standards and Federal guidelines on medical device reprocessing.12-14
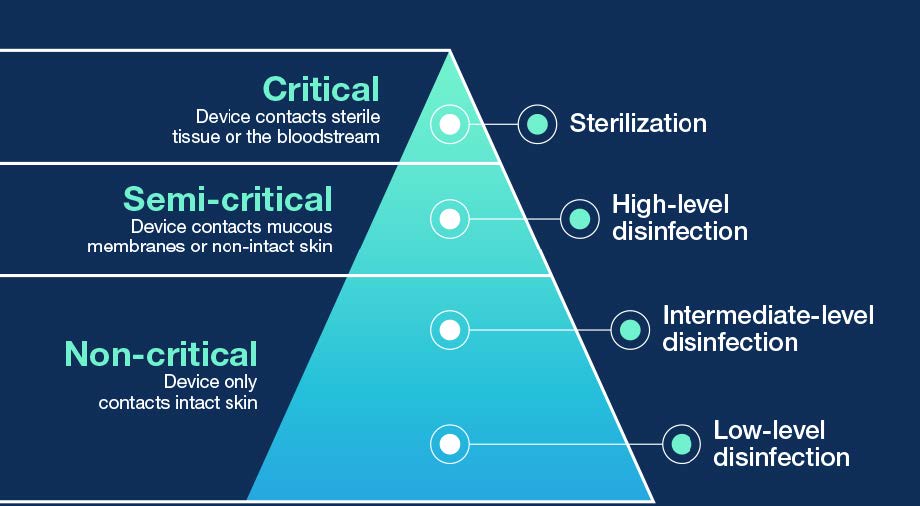
Figure 1. The Spaulding classification divides infection transmission risk based on the type of patient tissue the device will contact during use, which determines the level of disinfection.11-14 If sterilization of critical ultrasound probes is not possible, they can minimally undergo HLD and be used with a sterile sheath.12
Not all ultrasound procedures carry the same risk
Ultrasound is used in a diverse range of procedures with different levels of infection risk. For example, there are more than 140 ultrasound-guided percutaneous procedures (involving needle puncture of the skin), spanning at least 8 types of procedural category (Table 1). The wide variation in ultrasound-guided percutaneous procedures leads to variation in the patient sites contacted during use, as well as the risk of penetrating the sterile field. Many percutaneous procedures only involve contact between the probe and healthy, intact skin. Low-level or intermediate-level disinfection are appropriate in these cases. In other cases, there may be a risk of contact with broken skin during the procedure, meaning the device is semi-critical and requires HLD. Other times, the probe may break the sterile field or directly contact sterile tissue, such as the puncture site or adjacent sterile devices. In these scenarios, the device is critical and requires sterilization or, if sterilization is not possible, HLD. A notable example is biopsies, which are defined by the FDA as semi-critical or critical in nature, minimally requiring HLD.13
|
Specialty group |
Number of procedures |
|
Nerve block |
35 |
|
Aspiration |
27 |
|
Biopsy |
18 |
|
Drainage |
15 |
|
Vascular Access |
9 |
|
Injection |
9 |
|
Intraoperative |
8 |
|
Ablation |
6 |
|
Other |
13 |
|
Total |
140 |
Table 1. A selection of ultrasound-guided percutaneous procedures, collated from a review of published literature. The procedures can be broadly grouped into 8 different categories.
Use of a sheath does not change the level of reprocessing required
FDA-approved, single-use, sterile sheaths are also recommended for semi-critical and critical applications. However, it is important to note that the use of a sheath does not change the level of disinfection required.12,13 Condoms used on ultrasound probes can fail up to 13% of the time, while commercial covers fail up to 5% of the time.15 Sheaths and covers can also be punctured during needle-guided procedures. A surface ultrasound probe used in a central venous catheter (CVC) placement was found to have gouge marks on the transducer head, demonstrating that the needle had punctured the sheath and contacted the probe during the procedures.16 This means that, if the probe is not adequately reprocessed, microorganisms present on the transducer head could be directly introduced into the patient’s sterile tissue.
It only takes one poorly disinfected probe to put patients at risk
Two recent outbreaks in Europe and North America are a reminder about the importance of infection control in ultrasound. Sourced to contaminated lots of non-sterile ultrasound gel used in ultrasound-guided procedures, the outbreaks impacted hospitalized patients across multiple facilities.17,18 The U.K. Health Security Agency and the U.S. CDC issued safety alerts, calling upon immediate action to implement the routine use of sterile, single-use ultrasound gel in preparation for and during relevant procedures to prevent further adverse events. The CDC additionally called for a review of ultrasound probe reprocessing practices.18
Surface ultrasound probes can also carry risks. An intraoperative probe was the source of an outbreak among patients undergoing hepatic surgeries with the contaminated device.19 Improper probe reprocessing was identified as one of the factors contributing to the outbreak. Another study found ultrasound was associated with increased bloodstream infection risk when guiding central line insertions at the femoral and jugular sites. Contaminated gel was ruled out and confounding variables such as number of insertion attempts were controlled.20
Findings from the Joint Commission in 2020 showed that, out of 564 hospitals surveyed, 46.28% were non-compliant with activities around intermediate-level disinfection, high-level disinfection, and sterilization of medical devices.21 Surveyors observed transvaginal and transrectal probes that were reprocessed using low- to intermediate-level disinfection, and facilities not reprocessing ultrasound probes correctly after using a sterile sheath. Transvaginal and transrectal probes contact mucous membranes and are semi-critical devices, requiring HLD under Spaulding. To maintain patient safety, clinicians must be aware of the requirements of the Spaulding classification and able to correctly identify the level of reprocessing required by ultrasound probes.
trophon®2: leader in automated ultrasound probe HLD
Nanosonics trophon® technology is recognized as the world’s leading automated HLD solution for ultrasound probes. With over 31,000* units operating worldwide, over 102,000 patient procedures each day are protected from the risk of cross-contamination with trophon technology. The trophon family includes trophon EPR and trophon2 devices which share the same core technology of ‘sonically-activated’ hydrogen peroxide.
The trophon device goes beyond mandatory requirements and is demonstrated to eliminate an extended range of pathogens including multi-drug resistant bacteria and sexually transmitted pathogens. A study sampled transvaginal ultrasound probes after low-level disinfection with wipes, finding that more than 20% of probe heads remained contaminated with bacteria that should have been eliminated by LLD.4 In the same study, automated HLD with the trophon device successfully eliminated bacteria from both the probe heads and handles.
Nanosonics AuditPro provides facilities with the opportunity to further improve and standardize ultrasound infection control compliance across all ultrasound procedures. Nanosonics AuditPro includes a digital logbook system that links trophon2 cycle data to patient procedure information across every high-level disinfection cycle. The digital logbook replaces all manual documentation with a digital system that fits seamlessly into existing SOPs with no lost documentation or manual errors.
Together, trophon2 and AuditPro mitigate risk to patients and healthcare facilities, arming healthcare staff, IPs, and risk and quality managers with tools to drive compliance.
Reference:
- Frost & Sullivan: Healthcare Infrastructure and Procedural Volume for Ultrasound Imaging, 2018
- COVID-19 U.S. impact on antimicrobial resistance. 2022 Special Report.
- CDC, 2021 National and State Healthcare-Associated Infection Progress Report
- Buescher DL et al. 2016. Ultrasound Obstet Gynecol 47(5): 646-651.
- Oide S et al. 2019. J Med Ultrason 46(4): 475-479.
- Leroy S, et al. J Hosp Infect 2013;83(2): 99-106.
- M’Zali, F., et al. 2014. PLoS One 9(4): e93368
- Keys M et al. 2015. Crit Care Resusc 17(1): 43-46.
- Scott D et al. 2018. Ultrasound 26(3): 168-177.
- HPS, HFS 2016. NHS Scotland Guidance for Decontamination of Semi-Critical Ultrasound Probes
- Spaulding EH 1968. Chemical disinfection of medical and surgical materials. Disinfection, sterilization, and preservation. Lawrence C, Block SS. Philadelphia, Lea & Febiger: 517-531.
- CDC 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities.
- FDA 2019. Marketing Clearance of Diagnostic Ultrasound Systems and Transducers.
- FDA 2000. Content and Format of Premarket Notification [510(k)] Submissions for Liquid Chemical Sterilants/High Level Disinfectants.
- Basseal JM, et al. Infect Dis Health. 2020;25(2):77-81
- De Cassai A, Tonetti T. Central venous line placement and ultrasound probe damage: a word of caution. J Med Ultrasound 2019;27:110.
- Centers for Disease Control and Prevention. Multistate Outbreak of Burkholderia cepacia Infections Associated with Contaminated Ultrasound Gel.
- UK Health Security Agency National Patient Safety Alert NatPSA/2021/010/UKHSA.
- Gery A et al. 2021. J Hosp Infect 111: 184-188.
- Buetti N et al. 2020. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1817
- Joint Commission Online. August 11, 2021.
Nanosonics is an Australian infection prevention company that has successfully developed and commercialized a unique automated disinfection technology, trophon®. Since 2009, trophon EPR and trophon2 have redefined the standard of care in ultrasound probe reprocessing providing fail-safe ultrasound probe high level disinfection (HLD) to protect patients from the risk of cross contamination. Nanosonics AuditPro provides facilities and their broader organizations a digital workflow compliance management system by tracking ultrasound procedures across all departments to ensure probes have been appropriately disinfected according to the Spaulding classification. To learn more, visit https://www.nanosonics.us/
