During a May 19 webinar hosted by Becker’s Hospital Review and sponsored by Sandoz, three experts participated in a panel discussion and described the biosimilar landscape in the U.S. and shared opportunities to increase adoption of these products.
Panelists were:
- Sameer Awsare, MD, associate executive director, The Permanente Medical Group in Oakland, Calif.
- Christine Simmon, executive director, Biosimilars Council and senior vice president, policy & strategic alliances, Association for Accessible Medicines
- Len Arsenault, vice president, policy, medical and external engagement, Sandoz Inc.
Three key takeaways:
- Biosimilars could significantly reduce U.S. healthcare spending, but obstacles must be overcome. Biologic medicines, while highly effective, are a significant source of healthcare expenditures. A 2020 IQVIA report estimated that biologic medicines represent about 43 percent of invoice-level spending in the United States. Spending on these medicines has increased dramatically, with a 14.6 percent CAGR compared to 6 percent for the overall pharmaceutical market. “Biosimilars offer a promising solution, but the cost savings associated with these drugs haven’t kept pace with approvals,” Mr. Arsenault said. “In the last two years, the United States has lost more than $24 billion in potential savings because doctors haven’t used biosimilars. At the same time, American patients have foregone about $280 million potential out-of-pocket savings.” A variety of measures including public policy changes, enhanced biosimilars education and increased competition in the biologic marketplace are needed. Measures to prevent patent abuse, for example, would be helpful.
- The incentives embedded in federal policies aren’t currently aligned with increased biosimilar uptake. “Medicare Part B must be updated to reward providers that use lower-cost biosimilars. In addition, Medicare Part D needs to be modernized to encourage health plans and PBMs to favor lower-cost biosimilars,” said Ms. Simmon. Fortunately, legislators have introduced several proposals to address these challenges. The BIOSIM Act proposes an increase to the add-on that physicians receive when prescribing biosimilars. With the Shared Savings Program, physicians could earn part of the savings when prescribing a biosimilar that has a lower price than the reference product. In addition, a bill has been introduced to add a dedicated specialty tier to Medicare Part D, which would encourage plans to use biosimilars.
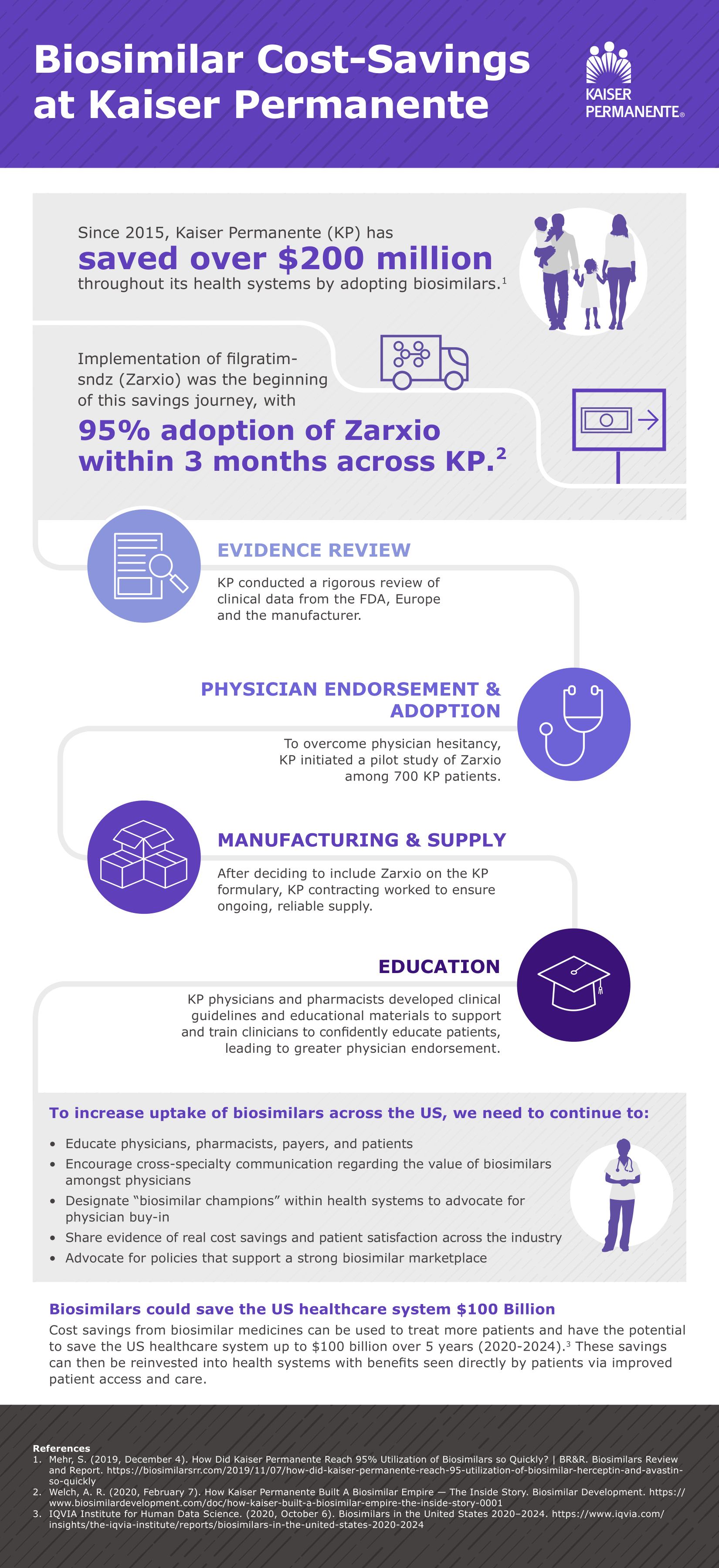
- To promote adoption of biosimilars, Kaiser Permanente focuses on evidence and education. When it comes to biosimilars, Kaiser Permanente is a case study in best practices. Before the organization adopts a biosimilar, physicians and pharmacists review the evidence. If they agree to use the product, the Kaiser Permanente contracting team negotiates a rate. By the time biosimilars reach the formulary committee, the appropriate clinicians have already vetted them. After approval by the formulary committee, Kaiser Permanente educates pharmacists, physicians, nurses and patients. The pharmacy research division also monitors patient outcomes and publishes pharmacovigilance data. “The biosimilar utilization rate at Kaiser Permanent is 82 to 95 percent compared to the reference biologic,” explained Dr. Awsare. “Over the last few years, our estimated savings has been about $200 million. We’ve used those resources to access new medications for our patients.”
To view a recording of the webinar, click here.