Imagine a nurse, let's call her Jill, who faces multiple scrub-ins everyday as part of her operating room role. She struggles with a rash on her hands and wrists that just won't go away, causing both physical and emotional trauma. These recurring and often painful skin conditions — which range from mild irritations to more serious reactions — can persist, despite the care taken by nurses and hospitals. Add to that the toll in sick leaves and absenteeism, and the cost of this condition can be painful to both staff and administration. Despite the care and costs, hospitals have not solved the problem. But there is an answer.
Our OR fictional nurse, Jill, suffers from a condition that is all too real in hospitals across North America: allergic contact dermatitis (Type IV allergy/chemical allergy). A chemical allergy is an expansive allergic condition which represents approximately 30 percent of occupationally induced skin diseases — and it is the second largest occupational disability reported to OSHA. While Jill’s hospital probably has an allergy management program, the focus is likely on allergenic risks that may be associated with the use of natural rubber (hevea brasiliensis) latex gloves. Meanwhile, the real cause of Jill's condition is allowed to roam hospitals at will, causing suffering to thousands of healthcare workers, costing an estimated $100,000 per year [1] to hospitals and burdening the national system to the tune of $1 billion per year.[2]
Presentation
A Type IV allergy is a reaction to specific allergens such as chemical accelerators used in the glove manufacturing process of both latex and synthetic gloves. Clinically, a Type IV allergy presents as a red, raised and palpable area at the area of contact with the glove, accompanied by subjective symptoms such as itching, burning and tingling. Additional symptoms include erythema, swelling, cracking, itching, weeping and dryness of the skin at the site of contact although dermatitis may extend beyond the area of contact.
The Type IV response begins when the antigens (such as residual chemicals leached from the glove in one's own perspiration) penetrate the skin, triggering the formation of T cells sensitized to the specific antigens. Repeated exposure to the antigen in allergic individuals results in the re-activation of sensitized T cells and the production of an inflammatory response causing the Type IV symptoms.
These effects typically appear anywhere from 6 to 48 hours following exposure to the antigen-containing product and can last up to four days.
Allergic contact dermatitis brings an even greater risk of bloodborne pathogen infection, because the body's most efficacious barrier — intact skin — becomes compromised. The breakdown of the dermis may also permit the passage of latex proteins into the body, thereby facilitating latex protein hypersensitivity in some individuals.
Occurrence and prevalence
While healthcare professionals are highly conscious of the Type I allergic risks linked to natural rubber latex gloves, the prevalence of Type IV allergies remains a mystery in many hospitals.
Clinical evidence [3] shows that Type IV allergies represent up to 28 percent of glove-related allergic reactions, with up to 90 percent of these Type IV allergies due to vulcanization accelerators used in the glove manufacturing process. The most frequent chemical issues are from accelerators which catalyze the cross-linking of elastomeric particles during production. [4]
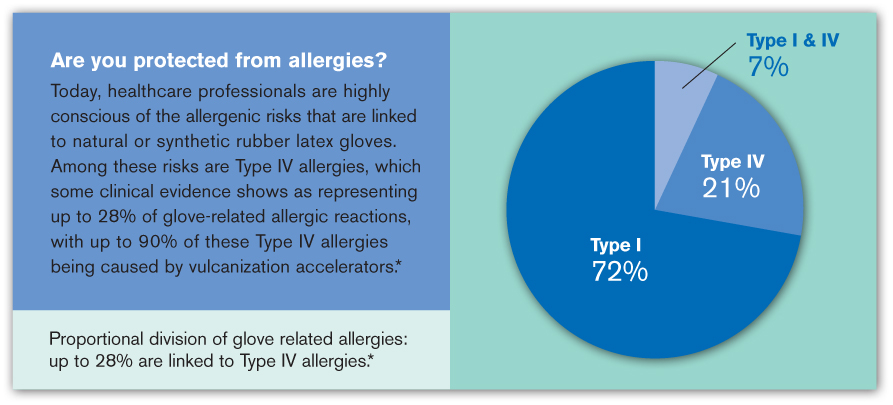
Clinical evidence shows that up to 28 percent of allergies are linked to Type IV allergies.
Dr. A. Heese, University of Erlangen, 1989-1992
Some individuals may also be sensitive to other substances associated with medical gloves:
• Lanolin, used as a glove softener by some manufacturers
• Polyoxypropyleneglycol, a coagulant used in the glove manufacturing process
• Coloring pigments, either organic or inorganic
• Quaternary ammonium compounds
• Antioxidants which are used to prevent the degradation of NRL products
• Preservatives
The cause
Chemical accelerators are traditionally used to accelerate the linkage of rubber molecules in natural or synthetic rubber. It's this accelerator group of chemicals (especially thiruams and carbamates) that induce the majority of the skin dermatitis reactions.
Chemical accelerators used in the manufacture of NRL and synthetic medical gloves transform the original raw liquid rubber state into a very thin, strong, elastic glove film and provide the glove the following:
• Cross-linking of the glove material to give strength to the glove
• Integrity to the glove during use
• Elasticity
• Stabilizization of the glove material for long-term storage
These chemicals, if not controlled, were recognized as a problem for healthcare workers more than 50 years ago, as reactions were occurring by exposure not only to latex gloves, but also to many other types of rubber products. The problem is basically an old dog showing off the same old tricks. And these tricks are going unrecognized by many healthcare workers.
Research conducted at the 2010 AORN Annual Congress showed that 53.9 percent of 954 respondents indicated that staff continued to experience allergy issues even after switching to non-latex gloves. [5]
That's because, as we've seen, chemical accelerators are generally used in the manufacturing of NRL and synthetic gloves. In fact, 78.3 percent of 947 respondents had experienced hand irritation that was determined to not be a Type I latex allergy.
Chemical allergies are not easily recognized, even by experienced healthcare workers like our fictional nurse, Jill. Take a look at these statistics from the AORN Annual Congress over two consecutive years.
• A significant percentage of OR nurses surveyed at the Congress in March 2009 (39 percent of 1,125 survey participants) and March 2010 (52.7 percent of 942 participants) demonstrated they did not know about chemical allergies.
• AORN survey results showed just 13.2 percent of 1,152 respondents have been tested by an allergist for a latex allergy. Only 4.5 percent of 1,149 respondents had been tested for Type IV chemical allergies.
The cost
While there are no reports that define the cost per facility related to allergic contact dermatitis, the national estimate for costs is $1 billion per year. (Note: To calculate your facility's financial risk for allergic contact dermatitis, visit AnsellProtects.com).
But with actual industry figures, it's easy to create a scenario that demonstrates the impact of possible costs to an average facility:
Let's assume a facility with 25 beds has each of their 12 affected nurses on sick leave once this year, putting absenteeism costs at approximately $34,000. Further, 25 percent of those nurses go on temporary disability due to continued exposure. Adding to this likelihood is the aging population in nursing and a disruption in the stratum corneum and skin barrier. This cost adds up to more than $10,600. Of these 25 percent, one head nurse must leave the profession, incurring a cost of $64,000 to replace that employee. The total cost for the above scenario is nearly $109,000 for just a one year time period.
Next steps
Chemical related skin allergies are inconvenient, painful, embarrassing and costly. But they are also preventable.
Here are some important steps to take in solving the mystery of Type IV chemical allergies:
• Get tested to determine what chemicals you are susceptible to. It's an important step and one that should not be put off. The costs are very high to you, your career and the level of care you can provide your patients.
• Ask your hospital administration about education programs to help you understand the causes and solutions to allergies and skin conditions related to your occupation.
• Not all accelerators are used in the manufacturing of medical gloves. Select gloves that do not use the accelerator you are allergic to.
• Look into new technologies that have led to the development of accelerator-free gloves. [6] These gloves are the latest innovation in the ongoing effort by glove manufacturers to provide effective barrier protection without causing allergic reactions.
For more information about Ansell products or to have the company's education and awareness programs at your facility to help educate staff about chemical allergies, call (800) 952-9916 or visit AnsellProtects.com
Footnotes:
[1] Financial Risks for Hospitals Due to Allergic Contact Dermatitis.
[2] CDC/NORA (National Occupational Research Agenda).
[3] Dr. A. Heese, University of Erlangen, 1989-1992.
[4] American Dental Association, Association Report – The Dental Team & Latex Hypersensitivity; JADA Vol. 130, February 1999.
[5] AORN Allergy Management Survey Results, April 2010.
[6] Understanding Medical Gloves & Differences. Sourced December 20, 2011 at http://en-medipart.com.my/knowledge-center/220-understanding-medical-gloves-a-differences.html
Our OR fictional nurse, Jill, suffers from a condition that is all too real in hospitals across North America: allergic contact dermatitis (Type IV allergy/chemical allergy). A chemical allergy is an expansive allergic condition which represents approximately 30 percent of occupationally induced skin diseases — and it is the second largest occupational disability reported to OSHA. While Jill’s hospital probably has an allergy management program, the focus is likely on allergenic risks that may be associated with the use of natural rubber (hevea brasiliensis) latex gloves. Meanwhile, the real cause of Jill's condition is allowed to roam hospitals at will, causing suffering to thousands of healthcare workers, costing an estimated $100,000 per year [1] to hospitals and burdening the national system to the tune of $1 billion per year.[2]
Presentation
A Type IV allergy is a reaction to specific allergens such as chemical accelerators used in the glove manufacturing process of both latex and synthetic gloves. Clinically, a Type IV allergy presents as a red, raised and palpable area at the area of contact with the glove, accompanied by subjective symptoms such as itching, burning and tingling. Additional symptoms include erythema, swelling, cracking, itching, weeping and dryness of the skin at the site of contact although dermatitis may extend beyond the area of contact.
The Type IV response begins when the antigens (such as residual chemicals leached from the glove in one's own perspiration) penetrate the skin, triggering the formation of T cells sensitized to the specific antigens. Repeated exposure to the antigen in allergic individuals results in the re-activation of sensitized T cells and the production of an inflammatory response causing the Type IV symptoms.
These effects typically appear anywhere from 6 to 48 hours following exposure to the antigen-containing product and can last up to four days.
Allergic contact dermatitis brings an even greater risk of bloodborne pathogen infection, because the body's most efficacious barrier — intact skin — becomes compromised. The breakdown of the dermis may also permit the passage of latex proteins into the body, thereby facilitating latex protein hypersensitivity in some individuals.
Occurrence and prevalence
While healthcare professionals are highly conscious of the Type I allergic risks linked to natural rubber latex gloves, the prevalence of Type IV allergies remains a mystery in many hospitals.
Clinical evidence [3] shows that Type IV allergies represent up to 28 percent of glove-related allergic reactions, with up to 90 percent of these Type IV allergies due to vulcanization accelerators used in the glove manufacturing process. The most frequent chemical issues are from accelerators which catalyze the cross-linking of elastomeric particles during production. [4]

Clinical evidence shows that up to 28 percent of allergies are linked to Type IV allergies.
Dr. A. Heese, University of Erlangen, 1989-1992
Some individuals may also be sensitive to other substances associated with medical gloves:
• Lanolin, used as a glove softener by some manufacturers
• Polyoxypropyleneglycol, a coagulant used in the glove manufacturing process
• Coloring pigments, either organic or inorganic
• Quaternary ammonium compounds
• Antioxidants which are used to prevent the degradation of NRL products
• Preservatives
The cause
Chemical accelerators are traditionally used to accelerate the linkage of rubber molecules in natural or synthetic rubber. It's this accelerator group of chemicals (especially thiruams and carbamates) that induce the majority of the skin dermatitis reactions.
Chemical accelerators used in the manufacture of NRL and synthetic medical gloves transform the original raw liquid rubber state into a very thin, strong, elastic glove film and provide the glove the following:
• Cross-linking of the glove material to give strength to the glove
• Integrity to the glove during use
• Elasticity
• Stabilizization of the glove material for long-term storage
These chemicals, if not controlled, were recognized as a problem for healthcare workers more than 50 years ago, as reactions were occurring by exposure not only to latex gloves, but also to many other types of rubber products. The problem is basically an old dog showing off the same old tricks. And these tricks are going unrecognized by many healthcare workers.
Research conducted at the 2010 AORN Annual Congress showed that 53.9 percent of 954 respondents indicated that staff continued to experience allergy issues even after switching to non-latex gloves. [5]
That's because, as we've seen, chemical accelerators are generally used in the manufacturing of NRL and synthetic gloves. In fact, 78.3 percent of 947 respondents had experienced hand irritation that was determined to not be a Type I latex allergy.
Chemical allergies are not easily recognized, even by experienced healthcare workers like our fictional nurse, Jill. Take a look at these statistics from the AORN Annual Congress over two consecutive years.
• A significant percentage of OR nurses surveyed at the Congress in March 2009 (39 percent of 1,125 survey participants) and March 2010 (52.7 percent of 942 participants) demonstrated they did not know about chemical allergies.
• AORN survey results showed just 13.2 percent of 1,152 respondents have been tested by an allergist for a latex allergy. Only 4.5 percent of 1,149 respondents had been tested for Type IV chemical allergies.
The cost
While there are no reports that define the cost per facility related to allergic contact dermatitis, the national estimate for costs is $1 billion per year. (Note: To calculate your facility's financial risk for allergic contact dermatitis, visit AnsellProtects.com).
But with actual industry figures, it's easy to create a scenario that demonstrates the impact of possible costs to an average facility:
Let's assume a facility with 25 beds has each of their 12 affected nurses on sick leave once this year, putting absenteeism costs at approximately $34,000. Further, 25 percent of those nurses go on temporary disability due to continued exposure. Adding to this likelihood is the aging population in nursing and a disruption in the stratum corneum and skin barrier. This cost adds up to more than $10,600. Of these 25 percent, one head nurse must leave the profession, incurring a cost of $64,000 to replace that employee. The total cost for the above scenario is nearly $109,000 for just a one year time period.
Next steps
Chemical related skin allergies are inconvenient, painful, embarrassing and costly. But they are also preventable.
Here are some important steps to take in solving the mystery of Type IV chemical allergies:
• Get tested to determine what chemicals you are susceptible to. It's an important step and one that should not be put off. The costs are very high to you, your career and the level of care you can provide your patients.
• Ask your hospital administration about education programs to help you understand the causes and solutions to allergies and skin conditions related to your occupation.
• Not all accelerators are used in the manufacturing of medical gloves. Select gloves that do not use the accelerator you are allergic to.
• Look into new technologies that have led to the development of accelerator-free gloves. [6] These gloves are the latest innovation in the ongoing effort by glove manufacturers to provide effective barrier protection without causing allergic reactions.
For more information about Ansell products or to have the company's education and awareness programs at your facility to help educate staff about chemical allergies, call (800) 952-9916 or visit AnsellProtects.com
Footnotes:
[1] Financial Risks for Hospitals Due to Allergic Contact Dermatitis.
[2] CDC/NORA (National Occupational Research Agenda).
[3] Dr. A. Heese, University of Erlangen, 1989-1992.
[4] American Dental Association, Association Report – The Dental Team & Latex Hypersensitivity; JADA Vol. 130, February 1999.
[5] AORN Allergy Management Survey Results, April 2010.
[6] Understanding Medical Gloves & Differences. Sourced December 20, 2011 at http://en-medipart.com.my/knowledge-center/220-understanding-medical-gloves-a-differences.html
