It is not outside the realm of possibility that Dr. David Fajgenbaum will follow in Dr. Barry Marshall’s footsteps in winning the Nobel prize in physiology. Marshall is cited for his 1982 discovery of "the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer disease." Perhaps his method is even more infamous than the discovery: Marshall, then perfectly healthy, swallowed a culture of the bacteria and intentionally developed gastritis. History of medicine is littered with similar examples of researchers heroically experimenting on their own bodies in the name of science. In David's case, it’s both in the name of science and in the name of life itself.
I met David when we were both graduate students at University of Pennsylvania's Wharton School. At the risk of sounding trite, his smile was infectious. He had shaggy, brown hair, and chiseled face, which exuded a permanent, welcoming warmth. That he suffered from an incurable, rare disease, idiopathic multicentric Castleman – an ambiguous condition straddling cancer and autoimmunity – which had repeatedly nearly killed him, was as unnerving as it was unexpected.

Far left, David Fajgenbaum in 2004, in a football drill at Georgetown University, and how he looked in February 2011, two weeks after his third flare-up of Castleman Disease.
The first sign something was wrong came in July 2010. David, then a medical school student, woke up in the middle of the night and proclaimed, "I think I’m dying." In his case, it wasn't much of a hyperbole. He was rushed to the hospital with multiple organ failure; weeks later there was still was no diagnosis. Myriad specialists remained flummoxed: in the interim, David had his last rites read to him bedside. Emerging from the brink of death, David soon relapsed; the cycle continuing 4 times, each time presumably the last. The experience transformed David from patient to assistant professor of medicine at Perelman School of Medicine at Penn, and undoubtedly one of the world’s preeminent Castleman researchers. The journey continues, but David's impact will be felt by millions of orphan disease patients worldwide. That he uses his own body as test-subject is an appropriate capstone.
Patients who suffer from rare, orphan diseases spend a lot of time hoping: hoping for a cure, yes, but smaller intermediary hopes too; hoping to raise enough money to fund research, hoping to find a relevant clinician, hoping necessary information flows amongst interested parties, never knowing where a helpful nugget may come from. In between all the hope, there is a lot of waiting. In David's words: "Despite being a physician, I didn’t fully understand the barriers that slowed down life-saving progress until I was dying from a rare illness. I received my last rites in 2010 while battling idiopathic multicentric Castleman’s disease and found that [the disease] had no FDA-approved therapies, a poorly understood model of pathogenesis, and a 65% 5-year survival rate."
Today, David and his team are upending decades of “traditional” orphan disease management. In the process, they are doing more than offering hope for millions of patients; they are creating the infrastructure that systemically shifts progress forward. Researchers, patients, healthcare companies, government officials: take note: "As a physician–scientist and patient with a deadly disease, I urge you to consider a more collaborative, strategic, and focused approach to accelerating research to save lives. We must work together to make every dollar and second count, because patients—like me—are waiting."1
An excerpt from an article Fajgenbaum and colleagues wrote in the Lancet Haematology describes the challenges and his approach:
"Despite technological advances and substantial investments of time and funding, many challenges exist for rare disease research and drug development. Basic disease mechanisms are often poorly understood, selection of suitable research participants can be difficult, and endpoints for clinical trial registration might not be established. Progress is further impeded by limited collaboration and data coordination, misaligned incentives, funding decisions made in isolation from community consensus, and inter-institutional barriers to tissue sharing. Although frequently used, off-label treatments are infrequently tracked, and thus valuable opportunities to build on observations are lost. Disease research organizations (DROs) make important contributions by funding research. However, the framework through which many DROs traditionally distribute funding can present challenges. DROs typically fundraise first and then invite researchers to apply through an RFP to use the funding for their specific purposes. In parallel, these organizations often provide patients with supportive resources and facilitate referrals to experts, who collect and store clinical data and biomaterials at their respective institutions. This model can in some cases result in organizations funding studies targeted at questions proposed by a small selection of researchers with requisite tissue samples rather than the studies that may have the highest impact on the field, which might require collaboration and sample sharing. A more efficient, collaborative, and consensus-driven framework than exists at present is needed to fully harness the opportunities afforded by technological advances for the approximately 7000 rare diseases and 350 million individuals afflicted globally."2
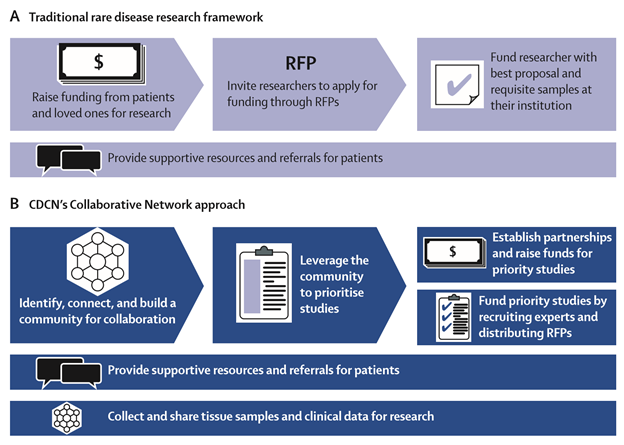
Figure: Traditional rare disease research framework (A) and the Castleman Disease Collaborative Network (CDCN)’s Collaborative Network approach (B)3
Not unlike most rare diseases, Castleman experts rarely collaborated, the community used different classifications, and no diagnostic criteria existed; the cause and pathogenesis of the idiopathic form was unknown. And so, in 2012, David assembled a group of physicians, researchers, and patients to create the Castleman Disease Collaborative Network (CDCN) in order to accelerate research through a targeted, collaborative, and patient-centric approach.
"The CDCN created a scientific advisory board, which now includes 28 members from eight countries. Physicians, researchers, patients, and loved ones joined the volunteer leadership team to advance research and support patients. In 2014, the CDCN submitted an application for a unique ICD-10 code for Castleman’s disease to facilitate epidemiological studies. The CDCN has hosted the four largest Castleman’s disease meetings and forged collaborations among a network of 317 physicians and researchers. In the past year, the CDCN has supported more than 6000 patients and loved ones through an online patient forum, patient summit, and website. Engagement of patients in the research process has aligned stakeholder incentives behind conducting research that will have the greatest impact on patients’ lives. To leverage the community to prioritize projects, the CDCN used online surveys, discussion boards, and patient feedback to crowdsource and establish the state of knowledge, a common classification system, and a list of prioritized studies called the International Research Agenda. Finally, the CDCN distributes funding through RFPs and strategically directed research grants whereby leading experts or contract research organizations are engaged to perform the highest priority projects."4
"In the past 3 years, the CDCN’s Collaborative Network approach has contributed to important progress for the field by increasing collaboration, focus, and community consensus. Combining findings from the published literature with communications among the CDCN community about hundreds of their unpublished cases"5 has garnered a breakthrough David is testing on himself: a drug called Sirolimus commonly prescribed for kidney transplant patients. The drug shuts down the body’s mTOR pathway, preventing the production of a protein, VEGF, which in turn, David hypothesized, should stabilize David’s immune system response and prevent another Castleman relapse. "I wouldn’t think I ever could have prescribed this to another patient, or told a patient to try it, because we just didn’t have very much data," David says. But so far, no relapse. And the data is accumulating.
While the fight is ongoing, David’s collaborative network approach unleashed an unprecedented level of coordination with tangible results: a new model of pathogenesis. Taking lessons from CDCN, let’s hope progress is accelerated for other diseases too.
Fajgenbaum has made tremendous progress, but a lot of work remains and he may relapse any day. Currently, he’s working tirelessly to acquire necessary patient samples and essential funding to conduct the studies that will save his life and others. To donate, please visit www.cdcn.org/donate-here
"Curing Castleman disease isn't Dave's hobby or hope, it's his calling and his ticket to survival. I felt inexplicably compelled to help in any way I could. It was the combination of his humble sincerity, passion, and relentless will to live."7
The writer is a healthcare services regulatory attorney and operator. He would like to thank David Fajgenbaum for allowing him to share this story, and for being one of the most decent people he has ever met.
References:
1. Dr. David Fajgenbaum
2. "The collaborative network approach: a new framework to accelerate Castleman's disease and other rare disease research" www.thelancet.com/haematology. Published online March 17, 2016 David C Fajgenbaum, Jason R Ruth, Dermot Kelleher, Arthur H Rubenstein Division of Hematology/Oncology, Orphan Disease Center, & Leonard Davis Institute for Health Economics (DCF) and Division of Endocrinology, Diabetes and Metabolism (AHR), Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA 02215, USA (JRR); and Faculty of Medicine, University of British Columbia, Vancouver V6T 1Z1, Canada (DK)
3. Id.
4. Id.
5. Id.
6. Alexendra Gilroy, Senior Product Manager, Genentech Inc.